Distribution of Electrons in Different Orbits (Shells)
Distribution of Electrons in Different Orbits (Shells): Overview
This topic covers concepts, such as Electrons Distribution in Different Orbits, Bohr and Bury Theory, Rules for Filling Electrons in Shells (Bohr-Bury scheme), Electron Distribution of First 18 Elements of Periodic Table, Electronic Configuration, Orbit Numbers, Maximum Number of Electrons in an Orbit & Maximum Number of Electrons in an Outermost Orbit etc.
Important Questions on Distribution of Electrons in Different Orbits (Shells)
Draw an electronic configuration of iron in .
An isoelectronic species are
The schematic atomic structure of four elements is given below. Observe and choose the right statement.
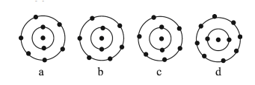
How many valence electrons present in ion?
The distribution of electrons in carbon is as follows:
The maximum number of electrons in a shell can be show by
Which one of the followings is correct electronic configuration of sodium?
Which element have two shells and both these shells are fulfilled with electrons?
Which will have electronic configuration ?
If electronic configuration of an atom is , then atomic number of the atom will be
What is the maximum number of electrons that can be accommodated in the outermost orbit?
An atom has an electronic configuration of . Which of the following elements will have properties similar to this?
The electronic configurations of four elements are:
Which of the above are present in the same period of the Periodic Table?
Identify the Mg2+ ion from the following figure where n and p represent the number of neutrons and protons respectively.
An atom has 2 electrons in K, 8 electrons in L and 6 electrons in M shell.The number of protons and electrons in divalent anion of the atom are respectively.
The electron distribution in a sulphide ion is
Which of the following correctly represents the electronic distribution in the sodium atom?
Which one of the following is the correct electronic configuration of aluminum?
